
Achieva Medical, an innovative medical solution service provider under Peijia Medical (9996.HK), is committed to innovation, research and development, and the manufacture of high-end cerebral vascular interventional products for hemorrhage, ischemia, and access.
The company was established in 2006.It owns the whole product pipeline and solution for hemorrhage, ischemia, and access.It has established GMP-compliant high-end medical device manufacturing (R&D) bases in Suzhou of China. and its sales channels cover more than 2,300 hospitals in 31 provinces (including municipalities and autonomous regions) in China.
 >
>

Listed in the "Suzhou Key Enterprise Intellectual Property Protection Directory."
Signed an exclusive distribution agreement with WarmSun Medical for flow-diverting stents.
Received the Outstanding Project Award at the Yangtze River Delta High-Value Patent Operation Competition.
Recognized as a Jiangsu Provincial Enterprise Technology Center.
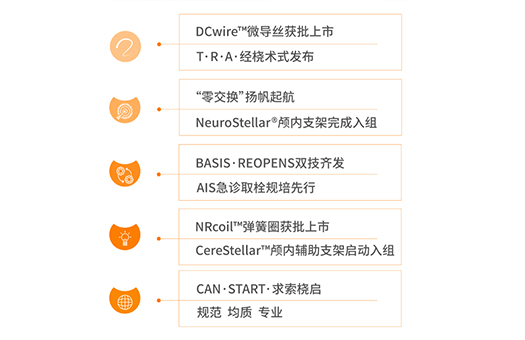
DCwire™ Microguidewire received market approval.
Launch of the T·R·A· Transradial Approach. Nationwide rollout of the "Zero Exchange" technology.
Successful completion of patient enrollment for the NeuroStellar® Intracranial Stent.
NRCoil™ Detachable Coil received market approval.
Initiation of clinical enrollment for the CereStellar™ Intracranial Support Stent.
Recognized as the Jiangsu Provincial Engineering Research Center for Neurointerventional Medical Devices.
Awarded the title of Jiangsu Provincial Specialized and Innovative SME.
Included in the Suzhou Gazelle Plan for high-growth enterprises.

Syphonet® Stent Retriever, Tethys AS® Aspiration Catheter, Fastunnel® Delivery Balloon Dilatation Catheter, and Fluxcap® Balloon Dilatation Catheter were approved for sale on the market.

The Achieva Cloud Home System was launched.
Jasper®SS Intracranial Electrolytic Detachable Coil and Heralder®DA Distal Access Guiding Catheter were approved for sale on the market.
The Company made the list of Enterprises Winning R&D grants from the Suzhou Municipal Government in 2021.
The 5th Achieva Medical Charity Campaign in Yushu was held online.
The first researchers' seminar on the intracranial stenting program was held.

SacSpeed® Balloon Dilatation Catheter was approved for sale on the market.
The Company was granted a production license to pilot the medical device registrant system in the Yangtze River Delta region.
Tethys™ Intermediate Guiding Catheter was approved for sale on the market.
Achieva Medical became affiliated with Peijia Medical Limited through a stock swap.
Yibida® Guiding Catheter was approved for sale on the market.

Achieva Medical passed the GMP on-site audit by ANVISA of Brazil.
Presgo® Mechanical Detachable Coil and Jasper® Intracranial Electrolytic Detachable Coil went on the market.

Achieva Medical became one of the first companies to have its application for innovative medical devices approved in 2017.
Presgo® Microcatheter and Presgo® Micro Guidewire entered the Chinese market.
The Achieva Medical (Suzhou) Co., Ltd. was founded.

The Suzhou Factory was set up.
Achieva Medical was honored as a "Shanghai High-Tech Enterprise".
The Jasper® Intracranial Electrolytic Detachable Coil was named a national key innovative product of China.
Our application for registration of Presgo® Mechanical Detachable Coil in Japan was approved.
Achieva Medical formally initiated the "Achieva Care" plan.
Achieva Medical was selected as a member of the China Association for Medical Devices Industry.
The market share of Jasper® Intracranial Electrolytic Detachable Coil in China surpassed 10%.
The sales of Jasper® Intracranial Electrolytic Detachable Coil exceeded one million for the first time.
Jasper® Intracranial Electrolytic Detachable Coil was launched.
Achieva Medical secured investment from Otsuka Pharmaceutical.
Achieva Medical was established in ZJ INNOPARK, Shanghai, China.
The predecessor of Achieva Medical was founded in San Diego, California.
Achieva medical adheres to the concept of "utmost devotion and reverence for life" and continues to create maximum value for customers, employees, shareholders and society.
Sink cutting-edge products where they are most needed

Gather little innovation, create the miracle of life
Do everything we can for a healthy China









